Soap and detergent
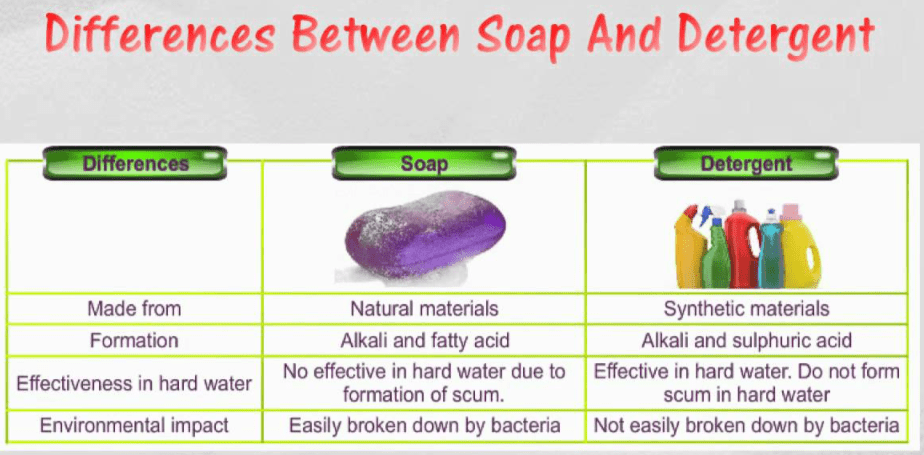
The Differences between Soap and Detergent is given here. It is very common for everyone to confuse the terms soap and detergent, since they both perform the same function before our eyes, eliminating dirt and bacteria.
keep reading…
Soap
Soap is a product used for personal hygiene and to wash some objects. It can be found in powder, cream, liquid, and pills. It comes from a mixture of fats and alkali.
It is usually a sodium or potassium salt that results from the chemical reaction between an alkali such as potassium or sodium hydroxide and a lipid. This reaction is known as saponification. The lipid can be of plant or animal origin. Soap is soluble in water.
Traditionally it is a solid material, but it can be found in a liquid form dissolved in water.
According to one of the legends about its origin, soap was discovered in Italy. It is said that animal sacrifices were performed on Mount Sapo, near Rome. The ashes and fat of the burned animals were washed into the river, where the slaves washed their masters’ clothes. It was soon discovered that soapy water washed clothes better and soap was created.
However, the oldest soap remains are of Babylonian origin and date back to 2800 BC. C.
Soaps clean fats in the presence of water because they are soluble in both compounds. Soap wet the grease and dissolves it in the water.
Detergent
It is a chemical substance with the property of dissolving dirt and impurities without scrubbing. Detergents can be defined as all those substances that have a cleaning or detergent effect on a surface. Any substance with the property of dissolving another substance is known as a detergent.
Most detergents are sodium compounds of substituted benzene sulfonate, called linear alkylbenzene sulfonates (LAS).
They have the wetting ability and can remove dirt while keeping residues suspended. They should also be easy to remove.
suggested video: Soap vs Detergent
Differences between Soap and Detergent
- Soaps share certain properties of detergents. It differs from these due to its surface activity, the property that allows them to dissolve in fat and water. This allows them to emulsify a water-insoluble substance.
- Detergent is a mixture of surfactants and other substances such as polyphosphates, silicates, perborates, and carbonates as well as substances such as enzymes, foams, colorants, and perfumes.